Chemicals and Services
Scale and Corrosion Inhibitor
Aside from scale, corrosion is also a stimulating problem in cooling tower. Corrosion cannot be completely stopped. However, there are several things you can do to help control or minimize corrosion. The following methods can be used to help control corrosion.
- Use corrosion resistant alloys. Once the cooling system is built, this becomes an expensive alternative.
- Adjust system pH. Increasing pH will reduce corrosion rate but will also increase the scaling potential.
- Apply protective coatings. These are generally not very effective because of the rough metal surfaces and difficulty of maintaining the integrity of the coating. Pre-treating system metallurgy with materials such as Nalprep after mechanical or chemical cleaning has been very effective at minimizing corrosion during system start-up.
- Use “sacrificial anodes”. These pieces of very reactive metal such as magnesium or zinc that are placed in heat exchangers. Since they are more reactive on the galvanic series than the heat exchanger the sacrificial anode will corrode instead of the heat exchanger.
- General Corrosion. Since all metal corrodes, the ideal situation is to take a small amount of metal evenly from throughout the system. This is called general corrosion. In general corrosion, the anode is very large.
- Localized Pitting Corrosion
- Galvanic Corrosion

Fouling is the accumulation of solid material, other than scale, in a way that hampers the operation of equipment or contributes to its deterioration. The following sources can produce these fouling materials in a cooling water system:
- Makeup water
- Air through cooling tower
- Internal contaminants
Each of these materials are suspended solids. They have a tendency to stick together and eventually settle out of the water. When this happens they form a deposit on metal surfaces in the cooling water system which interferes with the flow of water and the transfer of heat from the process.
Foulants are suspended solids such as, silt, sand, dirt, iron, corrosion products, microbiological growths, oil, process contaminants and carryover from a clarifier or lime softener. The factors which influence fouling can be summarized as follows:
- Water Characteristics. The potential for contamination from various sources.
- Water Temperature. Increasing temperature increases the fouling tendency.
- Water Flow Velocity. At low flow rates, less than 3.3 m/s, fouling occurs simply due to natural settling of suspended solids.
- Microbiological Growth. This is a major contributor to fouling. Microorganisms can grow on any surface. Since it is sticky it acts as a collection site for silt and dirt deposits. Some microorganisms are corrosive and contribute corrosion products to the fouling problem too. The free floating microbial masses can also act as foulants themselves.
- Process Contamination. Process materials that leak from heat exchangers can produce serious fouling problems. It can deposit in the system and it also can provide a food source for microorganisms.
Foulants will tend to form deposits in the hot areas of the system, the heat transfer zones, and in low flow areas of the cooling system. Heat exchangers with the cooling water on the shell side are the most vulnerable to fouling problems.
An effective way to prevent scales formation, corrosion, and fouling is application of chemical inhibitor. There are three general classifications for chemical corrosion inhibitors. Anodic inhibitors, cathodic inhibitors, general inhibitors.
- Anodic corrosion inhibitors such as chromates, nitrites, orthophosphates, silicates and molybdates work to stop the corrosion cell by blocking the anodic site.
- Cathodic corrosion inhibitors include bicarbonates, polyphosphates, polysilicates and zinc.
- General corrosion inhibitors such as soluble oils, tolyltriazoles and benzotriazoles protect metal by filming all surfaces whether they are anodic or cathodic.
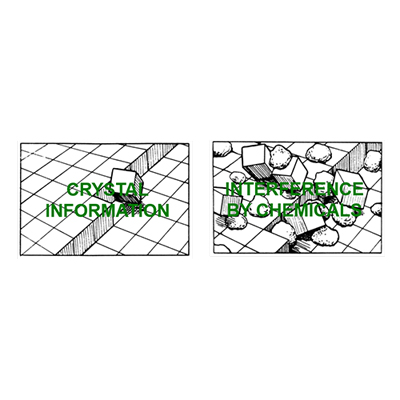
- Crystal Modification. Certain chemicals called organophosphates and organic dispersants are capable of distorting the crystal structure and preventing scale formation.
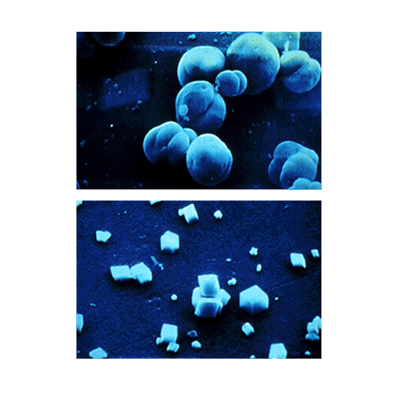
- Chemicals such as polyphosphates and anionic dispersants are able to form a complex with the troublesome minerals and prevent them from forming scale.
- Dispersancy. Compounds such as polyacrylates work because they are large molecules that impart a charge that causes the scale forming minerals to repel or stay away from each other.
- Liang Chi offers Hydralchem HC408 and Hydralchem HC508 scale and corrosion inhibitor chemicals for open type and closed type systems, respectively. The formulation uses combination of dispersant and anodic corrosion inhibitors to ensure the protection of heat exchangers despite the presence of our factors.

