Chemicals and Services
Descaling Compound
Mineral scale forms in the hot parts of the cooling system like heat exchangers and condensers. Failure to control mineral scale formation in a cooling water system will eventually result in shut down of the plant. Even before shut down of the system, the build-up of deposit reduces the ability of the water to take away heat from the process.
When scale forms, pitting type corrosion can occur under the deposit. This localized attack can quickly eat through the metal piping of the cooling system. The equipment may need to be totally replaced.
Corrosion on the other hand, is the mechanism by which metals are reverted back to their oxidized state. In nature, metals are mixed with oxygen. For instance, iron ore is iron mixed with oxygen. In the process of making steel the oxygen is removed to get the pure iron. This processed steel tends to rust, to react with oxygen and return to its natural state.
The corrosion process is just like the operation of a battery. There is an anode and a cathode, and an electrical circuit between them. In the corrosion cell, metal is lost at the anode.
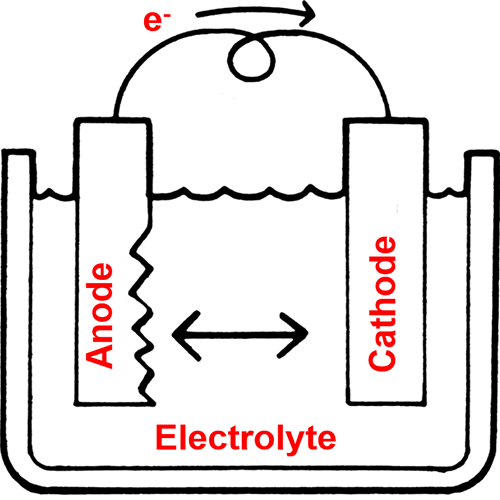
The amount of corrosion or the corrosion rate is affected by numerous factors.
- Water pH. The corrosion rate is dramatically affected by the cooling water pH. As the pH drops, the corrosion rate increases. A low pH allows reaction at the cathode to accelerate.
- Water Temperature. In general, for every 18°DF increase in water temperature the chemical reaction rate doubles. So, as the cooling water temperature increases, the corrosion rate increases.
- Dissolved Solids. It is the dissolved solids in the cooling water that complete the electrical circuit, from the cathode back to the anode. In general, the higher the dissolved solids, the higher the conductivity, the higher the corrosion rate.
- System Deposits. When scale, dirt, corrosion products, microbiological matter and other foulants deposit in the cooling system, they provide the perfect location for corrosion. Under these deposits anode sites for corrosion can develop. Under deposit corrosion can be severe and causes deep pits.
- Water Velocity. When water flow velocity slows down, it allows foulants to settle out of the water and form deposits. These deposits provide sites for under deposit corrosion. Deposits also interfere with the protective film provided by chemical treatment programs. Excessive flow velocity can cause erosion type corrosion. This is abrasion of the metal by the fast-moving solid particles in the water.
- Microbiological Growth. Microbio activity can increase corrosion in two ways. First, by depositing on metal surfaces, it allows under deposit corrosion to occur. Second, some microorganisms produce highly corrosive by-products, such as hydrogen sulfide. This results in severe pitting type corrosion.
There are several methods on how to prevent scaling and corrosion from depositing on systems, especially on heat exchanger tubes. These include:
- Limit the concentration of the scale forming minerals.
- Feed pH adjuster chemicals to vary pH and alkalinity.
- Make mechanical changes to the system design.
- Apply chemical scale inhibitors.

Liang Chi offers Hydralchem HC208, descaling compound for condenser cleaning, once scaling and fouling formed on tubes of condenser. The concentration and the duration of is carefully determined by the technical team to remove the depositions along the pipeline, while taking into consideration the possibility of corrosion (depending on the material of construction).pH monitoring is an essential part of the cooling tower water treatment system. If pH is less than 7, corrosion may occur. If pH is greater than 8, scaling may occur. As an initial line of defense, LCIPI-CSD also offers pH adjuster chemicals: Hydralchem HC308A to increase pH and HC308B to decrease pH, thus, maintaining the pH at recommended range of 7.0 to 8.0.In addition, its technical engineers give recommendations on additional lines of defense to further ensure that scales, corrosions and fouling will be eliminated. These include:
- Pre-treating the makeup water, through zeolites and other media filters, granular activated carbon filters, and water softener tanks.
- Bleeding off of the system to limit the concentration of the troublesome minerals.

